Research
The major research interest of K. Ogawa is whether Eph receptor tyrosine kinases and ephrin ligands may regulate cytoarchitecture in various tissues once development is completed. The roles of Eph receptors and ephrins have been extensively characterized in developing tissues. Different biological functions have been attributed to these proteins, including regulation of tissue-border formation, axon guidance, cell migration and vascular development. However, little is known about the localization and functions of these proteins in normal adult organs.
T. Nakajima studies the molecular and biochemical mechanisms in brain after global cerebral ischemia using rat models of cerebral ischemia. Cardiopulmonary resuscitation is conducted to rescue the patients subjected to cardiac arrest. Even when the restoration of spontaneous circulation is achieved by an appropriate cardiopulmonary resuscitation, about 40% of those who arrive to the hospital never regain consciousness, and about 30% of those awakened suffer post-cardiac arrest brain injury, including cognitive and motor deficits. Post-cardiac arrest brain injury is caused by global cerebral ischemia following cardiac arrest. At present, there is no useful pharmacological therapy for post-cardiac arrest brain injury in clinical setting. The global cerebral ischemia induced by the occlusion of common carotid artery and vertebral artery is good model of post-cardiac arrest brain injury. We study the pathophysiology of global cerebral ischemia using rat global cerebral ischemia model. Our research is a fundamental study for exploring a novel therapy for attenuating the post-cardiac arrest brain injury.
Global cerebral ischemia model
Four-vessel occlusion (4-VO) in the rat has been extensively used for global cerebral ischemia models. Four-VO is accomplished by the coagulation of the vertebral arteries and temporal ligation of the common carotid arteries. Briefly, the bilateral vertebral arteries are permanently occluded by electrocauterization under an anesthesia. After allowing a 24 h recovery, rats are anesthetized with 1.5% halothane in 30% oxygen and 70% nitrous oxide, and ischemia is induced by occluding the bilateral common carotid arteries with aneurysm clips. Sham-operated animals are treated similarly to those subjected to ischemia, except for the occlusion of the common carotid arteries.
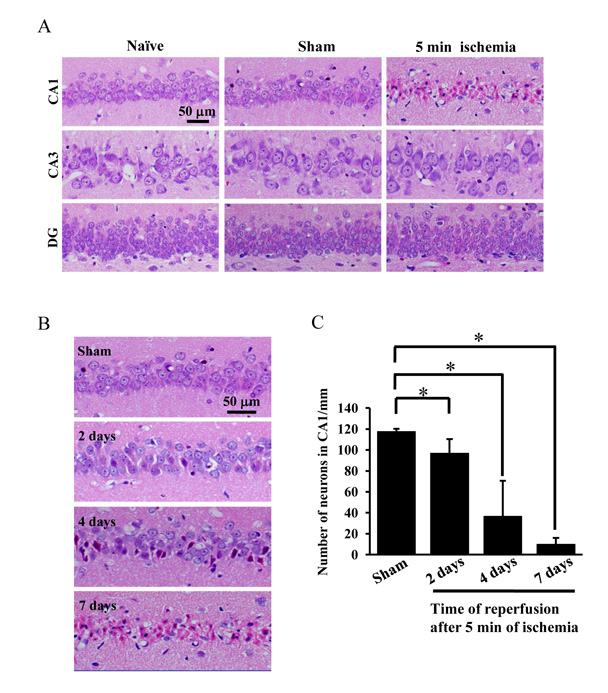
Histological changes in the hippocampus after 5 min of ischemia.
(A) Photographs of hematoxylin/eosin staining sections from naïve, sham-operated rats and
rats subjected to 5 min ischemia followed by 7 days of reperfusion. (B) Photographs of
hematoxylin/eosin staining sections from sham-operated rats and rats subjected to 5 min ischemia followed by 2 days, 4days and 7 days of reperfusion. (C) The changes in the number of pyramidal neurons per 1-mm length were tested by two-tailed unpaired Student’s t test. Data are expressed as the mean ± standard deviation (SD). *Statistically significant difference from rats subjected to sham-operation (P < 0.05); n = 4. DG dentate gyrus. Sham: sham operation.
Transforming growth factor-β (TGF-β) superfamily
Transforming growth factor-β (TGF-β) superfamily proteins, including TGF-βs, activins, and bone morphogenetic proteins (BMPs), regulate mutiple cellular functions in both fetal and adult tissue. TGF-βs inhibit the proliferation of epithelial cells and lymphocytes, activins regulate the release of follicle-stimulating hormone and the differentiation of erythrocytes, and BMPs contribute to the differentiation of osteoblasts. In brain, TGF-βs, activins, and BMPs are involved in several physiological functions. TGF-βs function to inhibit the synapse formation (Glia. 2014. 62:1917-1931.), control extracellular glutamate homeostasis, and extra-synaptic calcium responses (Glia. 2013. 61:985-1002.). Activin has been reported to be involved in memory maintenance (Learn Mem. 2010. 17:176-185.), possibly through the regulation of dendritic spine morphology (J Cell Sci. 2007. 120:3830-3837.). BMP is associated with the control of neurogenesis in the subventricular zone of the brain (Neuron. 2000. 28:713-726.). Smad proteins are known to transduce the actions of the TGF-β family. To date, eight Smad proteins have been characterized: Smad1, -2, -3, -4, -5, -6, -7, and -8. These are divided into 3 subclasses based on their function and structure: receptor-regulated Smads (R-Smads), the common partner Smad (Co-Smad), and inhibitory Smads (I-Smads). When TGF-βs, activins, and BMPs bind to their specific receptors on the cell surface, the receptors activate R-Smads via carboxy-terminal phosphorylation. R-Smads include Smad1, -2, -3, -5, and -8. The only known mammalian Co-Smad is Smad4, which forms a complex with phosphorylated R-Smad before translocating to the nucleus. I-Smads include Smad6 and Smad7 and inhibit the activation of R-Smads by inducing degradation of the receptors or by competing with the R-Smads for type I receptor binding. Receptors for TGF-βs, activins, and BMPs consist of 2 types of transmembrane serine/threonine kinase receptors: type I and type II. Seven type I receptors (activin receptor-like kinases (Alk) 1-7) and 4 type II receptors (TGF-β receptor II (TβRII), activin receptor IIA (ActRIIA), -IIB (ActRIIB), and BMP receptor II (BMPRII)) have been characterized. TβRII forms a complex with Alk1 or Alk5. ActRIIA and ActRIIB form a complex with Alk2, -4, or -7. Similarly, BMPRII forms a complex with Alk2, -3, or -6. There is a difference in the affinity for ligands between type II receptors. TβRII and BMPRII have an affinity for TGF-β and BMP, respectively. ActRIIA/IIB has an affinity for activin as well as BMP. When type II receptors bind to their ligands, they activate type I receptors, which in turn phosphorylate R-Smads. Alk4, -5, and -7 phosphorylate Smad2 and Smad3, while Alk1, -2, -3, and -6 phosphorylate Smad1, -5, and -8. 
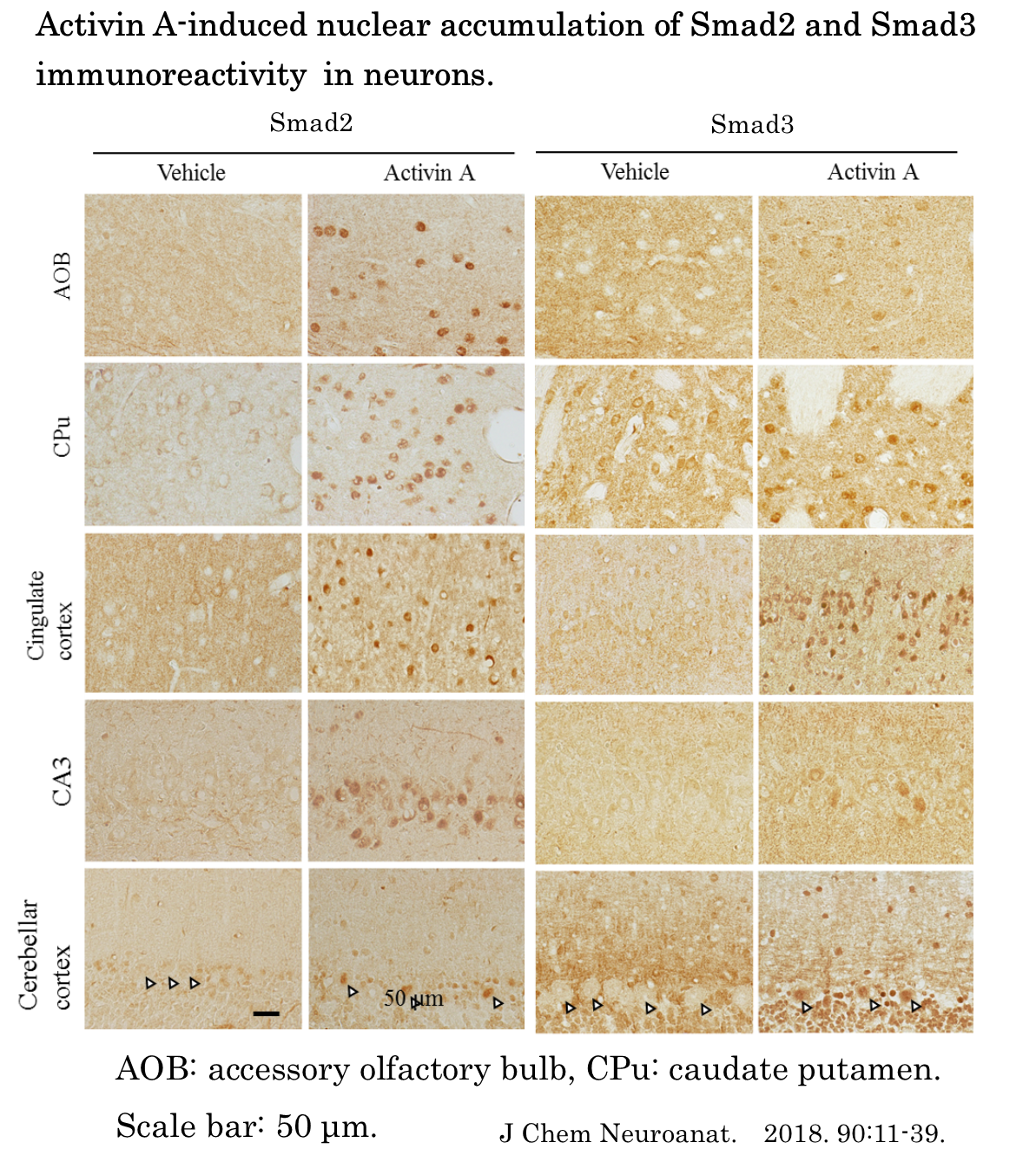
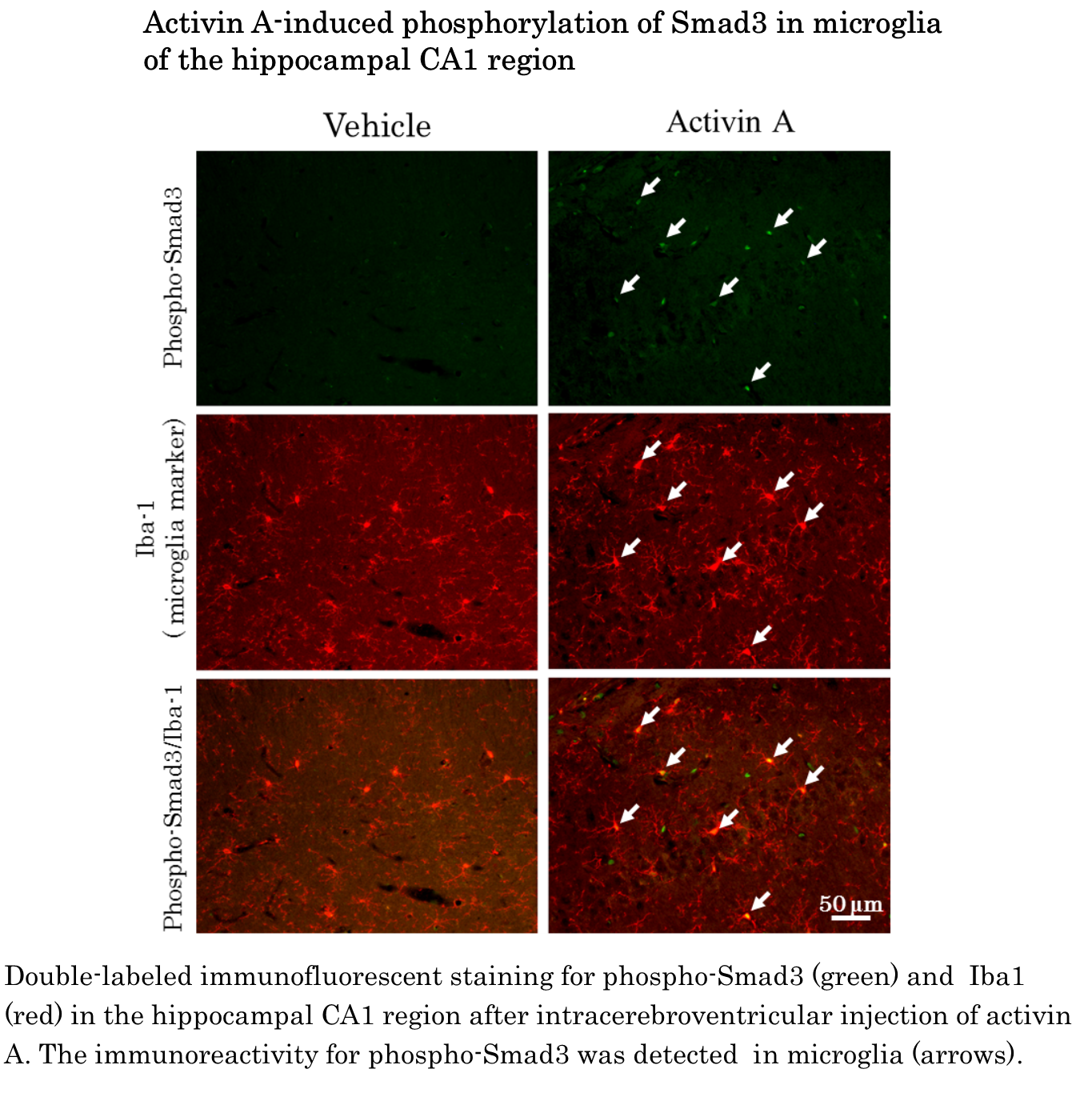
There has been accumulating data on the distribution of its ligands and receptors in brain. However, little is known about the distribution pattern of Smads throughout the brain. In this study, we examined the distribution of Smad1, -2, -3, -4, -5, and -8 mRNA in the rat brain using in situ hybridization (ISH). We also investigated the nuclear translocation of Smad1, -2, -3, -5, and -8 proteins or phosphorylation of Smad3 and Smad1/5/8 following intracerebroventricular
injection of TGF-β1, activin A, or BMP6 by immunohistochemistry to determine whether TGF-β, activin, and/or BMP regulate signaling Smads in the rat brain.
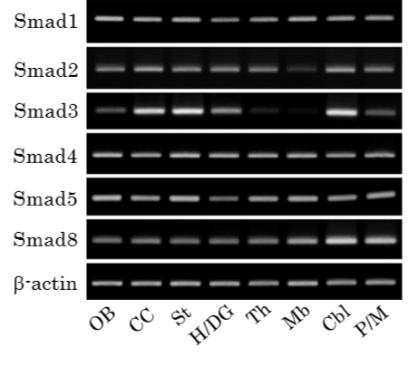
RT-PCR analysis of the expression of Smad1, -2, -3, -4, -5, and -8 mRNA in different brain regions.
Representative PCR products in different brain regions. OB: olfactory bulb, CC: cerebral cortex, St: striatum, H/DG: hippocampus and dentate gyrus, Th: thalamus, Mb: midbrain, Cbl: cerebellum, P/M: pons and medulla oblongata.
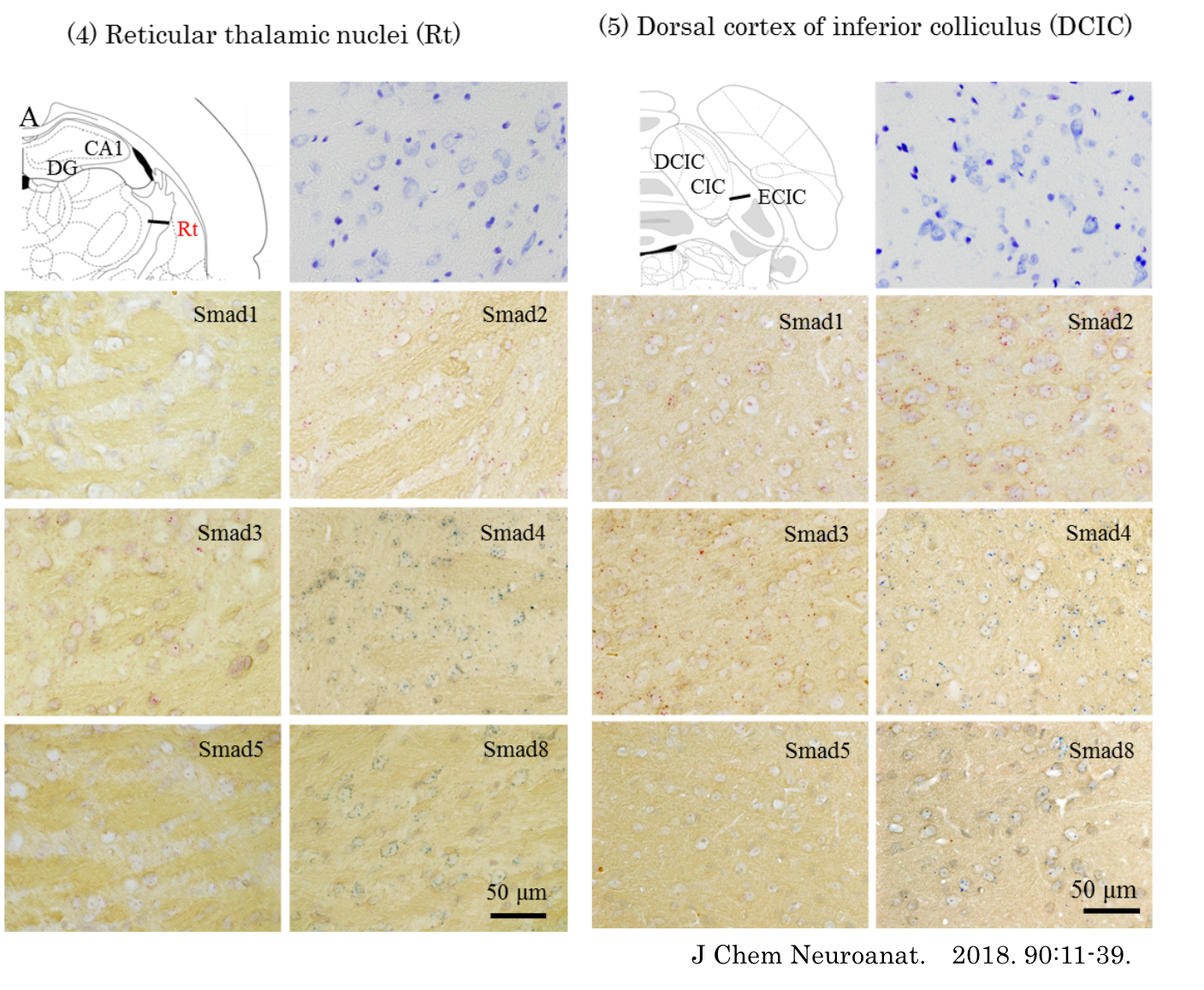
Representative photographs of in situ hybridization signals.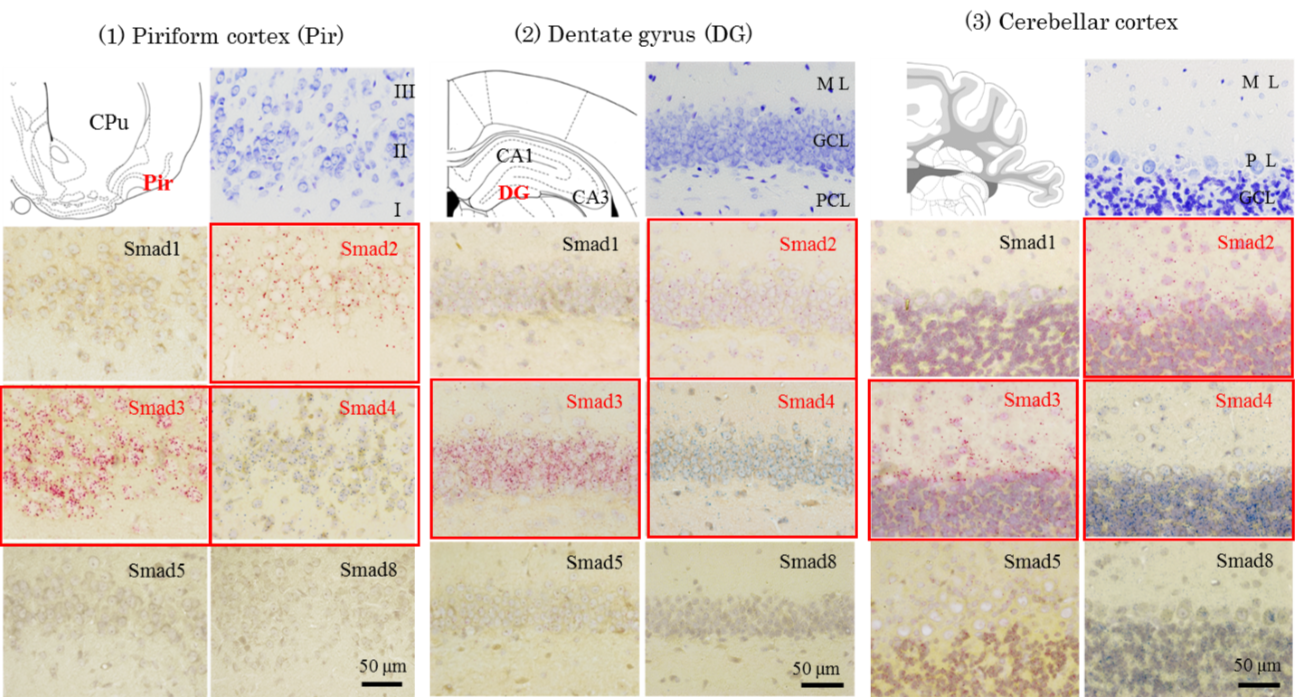
 Schematic representation of the distribution of ISH signals for Smad1, -2, -3, -4, -5, and -8 mRNA in the rat brain. The drawing of brains are adopted from Paxions and Watson (2009). ISH signals are represented by red or blue dots in appropriate densities.
Schematic representation of the distribution of ISH signals for Smad1, -2, -3, -4, -5, and -8 mRNA in the rat brain. The drawing of brains are adopted from Paxions and Watson (2009). ISH signals are represented by red or blue dots in appropriate densities.
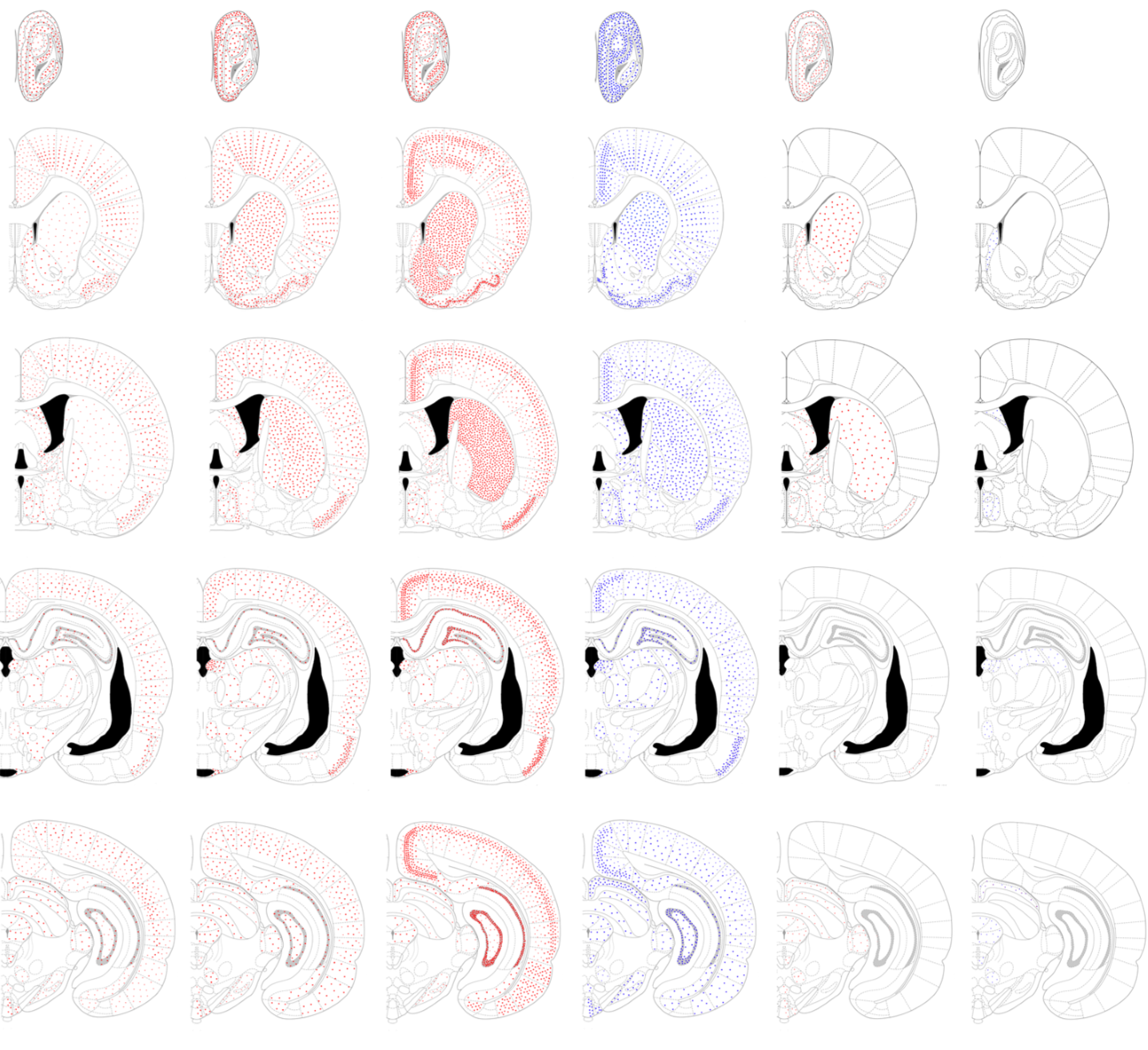 Smad1, -2, -3, -4, -5, and -8 mRNA were widely expressed in the rat brain whilst the expression pattern of different type of Smad mRNA varied between brain regions. We found that the ISH signals for mRNAs of different types of R-Smads had a unique distribution pattern. The signals for Smad1 mRNA were detected across several brain regions, but not the olfactory tubercle and cerebellar nuclei. When detected, all Smad1 signals were either weak or moderate. Signals for Smad2 mRNA were abundantly observed in the olfactory bulb, piriform cortex, basal ganglia, cingulate cortex, epithalamus including pineal gland and medial habenular nuclei, hypothalamus, inferior colliculi of the midbrain, a part of pontine nuclei, cerebellar cortex and choroid plexus. As compared to these regions, the signal intensity for Smad2 mRNA was reduced throughout the thalamus, except for the epithalamic regions, in the midbrain except for inferior colliculi, and most of the pons and medulla oblongata. Signals for Smad3 mRNA were abundantly observed in the olfactory bulb, olfactory tubercle, piriform cortex, entorhinal cortex, amygdala, basal ganglia, hippocampus, dentate gyrus, cingulate cortex, cerebral neocortex, hypothalamus, inferior colliculi of the midbrain, cerebellar cortex, and choroid plexus. In contrast, weak signals for Smad3 mRNA were observed in the thalamus, midbrain structures except for inferior colliculi, pons, and medulla oblongata. In some midbrain nuclei, Smad3 mRNA was not detected. These results suggest that the expression of Smad2 and Smad3 is site-selectively regulated in brain. Weak signals for Smad5 and Smad8 mRNA were detected in a small number of brain regions. These results suggest that the expression of Smad5 and Smad8 is strictly regulated within the brain. Smad4 mRNA were detected in all regions examined in this study.
Smad1, -2, -3, -4, -5, and -8 mRNA were widely expressed in the rat brain whilst the expression pattern of different type of Smad mRNA varied between brain regions. We found that the ISH signals for mRNAs of different types of R-Smads had a unique distribution pattern. The signals for Smad1 mRNA were detected across several brain regions, but not the olfactory tubercle and cerebellar nuclei. When detected, all Smad1 signals were either weak or moderate. Signals for Smad2 mRNA were abundantly observed in the olfactory bulb, piriform cortex, basal ganglia, cingulate cortex, epithalamus including pineal gland and medial habenular nuclei, hypothalamus, inferior colliculi of the midbrain, a part of pontine nuclei, cerebellar cortex and choroid plexus. As compared to these regions, the signal intensity for Smad2 mRNA was reduced throughout the thalamus, except for the epithalamic regions, in the midbrain except for inferior colliculi, and most of the pons and medulla oblongata. Signals for Smad3 mRNA were abundantly observed in the olfactory bulb, olfactory tubercle, piriform cortex, entorhinal cortex, amygdala, basal ganglia, hippocampus, dentate gyrus, cingulate cortex, cerebral neocortex, hypothalamus, inferior colliculi of the midbrain, cerebellar cortex, and choroid plexus. In contrast, weak signals for Smad3 mRNA were observed in the thalamus, midbrain structures except for inferior colliculi, pons, and medulla oblongata. In some midbrain nuclei, Smad3 mRNA was not detected. These results suggest that the expression of Smad2 and Smad3 is site-selectively regulated in brain. Weak signals for Smad5 and Smad8 mRNA were detected in a small number of brain regions. These results suggest that the expression of Smad5 and Smad8 is strictly regulated within the brain. Smad4 mRNA were detected in all regions examined in this study.
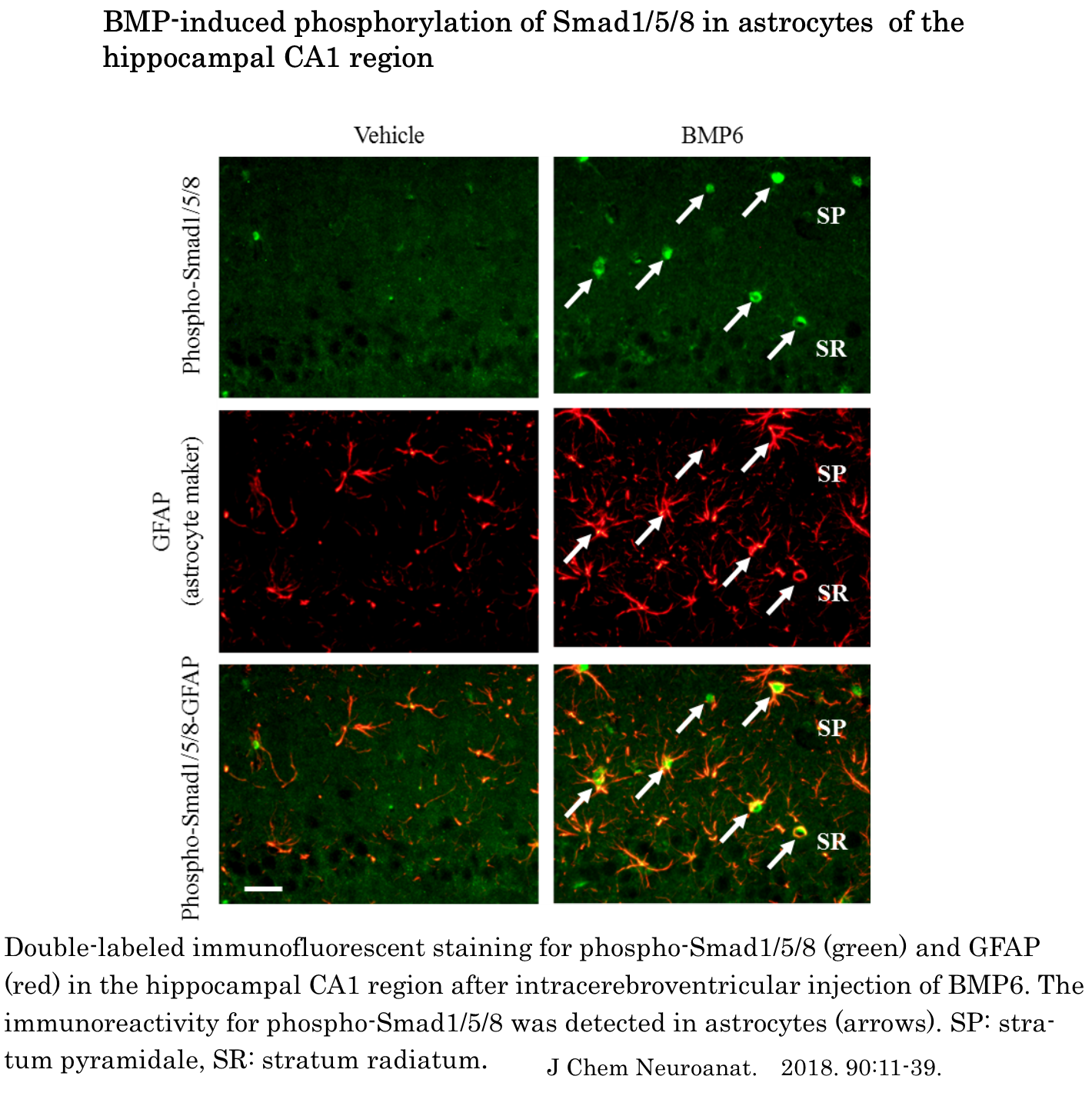
The present study is the first report that comprehensively shows the expression pattern of Smad1, -2, -3, -4, -5, and -8 mRNA in the rat bran. The major findings of the present study were as follows. Firstly, we showed that Smad1, -2, -3, -4, -5, and -8 mRNA were widely expressed in the rat brain whilst the expression pattern of different type of Smad mRNA varied between brain regions. Secondly, injection of activin A into the lateral ventricular led to accumulation of Smad2 and Smad3 immunoreactivity in the nucleus of neurons and induced the phosphorylation of Smad3 in microglia. Thirdly, the intracerebroventricular injection of BMP6 induced the phosphorylation of Smad1/5/8 in astrocytes. The present results suggest that activin-Smad signaling plays an important role in function of neurons and microglia and that astrocytes play a role in some physiological function in response to BMP in adult rat brain.